Abstract
Pharmacological targeting of cellular pathways critical to Chronic lymphocytic leukemia (CLL) survival is effective, but costly and associated with long-term toxicity and resistance. Chimeric antigen receptor (CAR) T cell therapy has been promising in CLL treatment showing durable remission, but only ¼ CLL patients can achieve complete remission with persistent effect featured by early memory T cells. This disparity is attributed to T cell-intrinsic defects or tumor-mediated immunosuppression. However, the mechanisms by which leukemic cells impact CAR T cell potency are poorly understood.
Results: In this study, we used second-generation CD19- and ROR1-directed of CAR T cells with a 4-1BB intracellular signaling domain. To recapitulate CLL-derived T cell defects, we developed an in vitro model of chronic antigen stimulation using CAR T cells recursively exposed to primary CLL cells. An irradiated K562-based artificial antigen-presenting cell (aAPC) expressing corresponding CAR target antigen was used as a positive control. Both CAR T cells were stimulated potently by aAPCs, showing robust proliferation as reported, while CAR T cells stimulated by CLLs showed significantly less proliferation.
Next, we demonstrated that direct contact with CLL cells did not irreversibly impair CAR T cell function, but instead insufficiently activated these cells, which could be rescued by antigen stimulus. In fact, in admixtures of CLLs and aAPC, 25% aAPC is sufficient to robustly stimulate and enhance CAR-ROR1 T cell proliferation as well as cytokine secretion, compared to the group with CLL only. Notably, IL-2 production decreased with increasing number of CLL cells in co-culture.
To assess the effects of IL-2 reduction, we supplemented exogenous IL-2 to CAR T cells in CLL co-culture. IL-2 supplementation can overcome impaired CAR T cell proliferation in a dose-dependent manner. Furthermore, IL-2 was detected significantly higher than supplemented level, showing that cytokine production ability of CAR T cells was partially restored in IL-2 supplemented cultures. On the other hand, IL-2 also affects phenotype of CLL cells by enhancing expression of co-stimulatory molecules CD80 and CD86, which can improve CAR T cell response.
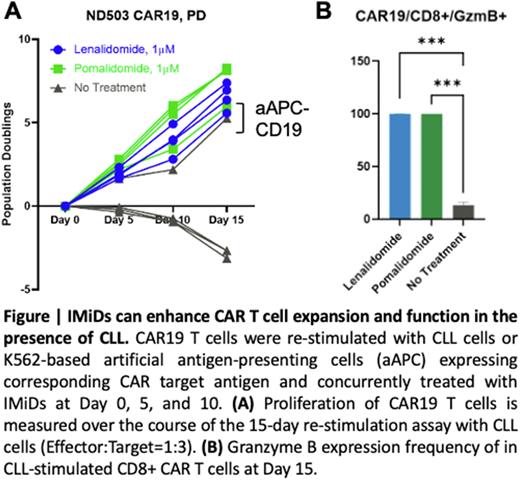
In line with this, immunomodulatory drugs (IMiDs) lenalidomide and pomalidomide have previously been demonstrated to upregulate co-stimulatory molecules on CLL and restore T-cell function. IMiDs boost T-cell function by redirecting E3 ligase subunit cereblon to target IKZF family transcription repressors Ikaros (IKZF1) and Aiolos (IKZF3) for degradation, leading to IL-2 upregulation. So we sought to test if IMiDs can improve CAR T cell response to CLL. We first determined the optimal IMiDs treatment conditions by titration on CAR T cells. Our preliminary data validated that lenalidomide or pomalidomide treatment restores CAR19 and CAR-ROR1 proliferation when repeatedly exposed to primary CLL cells. IMiDs can enhance CAR T cell differentiation (higher frequency of effector memory T cells), activation (HLA-DR, CTLA-4), proliferation (Ki67), and effector function (Granzyme B) when repeatedly stimulated by primary CLL cells. Interestingly, PD-1 decreased upon IMiDs treatment at Day 15 in CD8+ CAR T cells, indicating less exhausted phenotype.
Conclusions: Overall, our study unveiled that in CLL, activation defect of 4-1BB, CD3-signaling anti-CD19 CAR T cells was attributable to low levels of co-stimulatory molecules on CLL cells and can be effectively overcome via upregulation of co-stimulation. Our preliminary data suggested that CLL resistance to CAR T cell can be rescued by concurrent treatment with IMiDs. This study provides a potential translational approach to overcome the bottleneck of CLL treatment by CAR T cells.
Disclosures
Sun:Genmab: Research Funding. Wiestner:AstraZeneca: Research Funding; Abbvie: Research Funding; Acerta Pharma: Research Funding; GenMab: Research Funding; Merck: Research Funding; Nurix: Research Funding; Pharmacyclics: Research Funding; Verastem: Research Funding. Melenhorst:Novartis: Patents & Royalties: Biomarkers predictive of therapeutic responsiveness to chimeric antigens; Novartis: Patents & Royalties: Methods for improving the efficacy and expansion of immune cells; Chroma Medicine: Consultancy; Kite Pharma: Consultancy; Poseida Therapeutics: Consultancy; IASO Biotherapeutics: Consultancy, Research Funding; Novartis: Patents & Royalties: Methods of making chimeric antigen receptor - expressing cells; Novartis: Patents & Royalties: Methods for improving the efficacy and expansion of chimeric antigen receptor.
Author notes
∗Asterisk with author names denotes non-ASH members.